C. difficile and the Cobra Effect: When Good Intentions Backfire
By Natalia Soledad Ríos Colombo, PhD
Have you ever heard of the cobra effect? It’s a term used to describe situations where a well-intentioned action backfires, producing the exact opposite of what was intended. The term originates from an anecdote in colonial India. Concerned about the high number of venomous cobras in Delhi, the British government offered a bounty for every dead cobra brought to them.
At first, the program seemed successful, but eventually, people began breeding cobras to collect the reward. When the government realized this, the bounty program was cancelled, and the now-worthless cobras were released. The result? An even larger cobra population than before.
Reading about the cobra effect instantly reminded me of an experiment I’ve been struggling with for weeks.
How do cobras connect to C. difficile?
C. difficile infection (CDI) is a common cause of gut inflammation and diarrhoea, particularly in healthcare settings. It primarily affects immunocompromised patients, who often undergo multiple rounds of antibiotic treatment. Antibiotics are well-known to disrupt the gut microbiome, creating an environment where C. difficile—a bacterium that can sometimes coexist harmlessly—overgrows and causes infection.
The standard treatment? More antibiotics. This creates a vicious cycle, often leading to recurring C. difficile infections. It’s a nightmare for patients, their families, and healthcare systems.
What about faecal microbiota transplants (FMTs)? These have shown great success in restoring gut microbial diversity and preventing C. difficile recurrence. However, the patients most affected by CDI—older adults and the immunocompromised—are often not eligible for FMTs due to their weakened immune systems.
This raised a question for our team: is there an alternative to the commonly used antibiotics?
Bacteriocins to the rescue?
As someone who has spent most of my (academic) life studying small antimicrobial peptides called bacteriocins, I might be biased, I tend to think they’re the solution to everything. A lot of bacteriocins are naturally produced by members of the human microbiome, and they typically target specific bacterial populations, helping the producing bacteria survive and compete in the gut.
This specificity is why we thought bacteriocins might offer a promising alternative to the broad-spectrum antibiotics commonly used in clinical practice.
But, as explained before, C. difficile pathogenesis is closely tied to the gut microbiome context. Studying the microbiome is extremely difficult—it’s complex, dynamic, and incredibly diverse. When using complex microbial communities, like human faecal samples, the variability between individuals becomes a major challenge for interpreting the data.
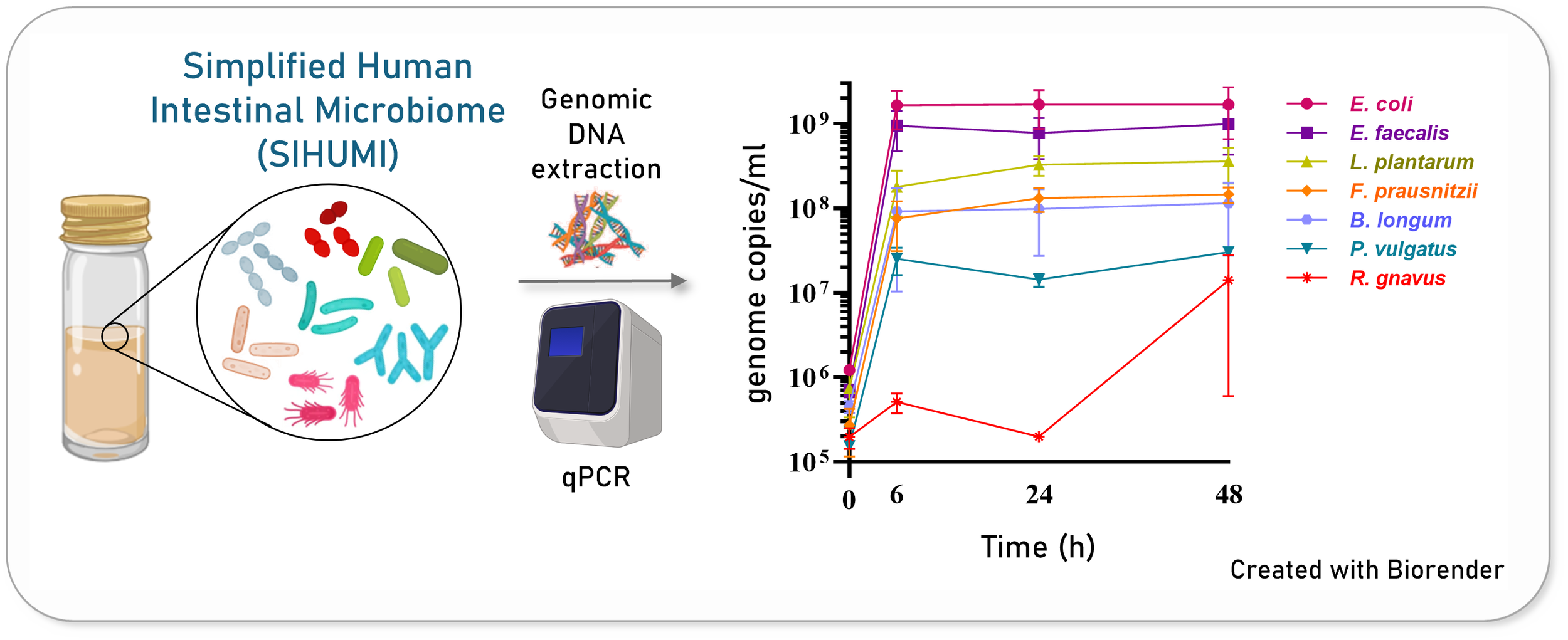
Thankfully, in our lab, we work with a Simplified Human Intestinal Microbiome (SIHUMI) model—a defined consortium of bacterial strains isolated from the human gut. SIHUMI allows us to obtain reproducible data in a controlled experimental setting. By including a pathogenic C. difficile strain, we transformed SIHUMI into an in vitro model of CDI.
The Experiment
Our first step was engineering a set of Lactococcus lactis strains to produce different combinations of bacteriocins, along with a non-producer strain as a negative control. When we tested the inhibitory activity of each strain against C. difficile, one particular combination showed synergy, displaying a high anti-C. difficile activity. I can’t describe how thrilled I was to see those results.
Next, we tested how this L. lactis strain performed against C. difficile in the context of our gut microbial community, SIHUMI. This is where things started to go wrong. Surprisingly, our leading bacteriocin-producing candidate ended up increasing C. difficile levels in SIHUMI, despite showing great inhibition when tested against C. difficile on its own.
At this point, picture me crying at the lab bench. If you’ve ever been a PhD student, you probably know the feeling. And then it hit me: I was experiencing the cobra effect firsthand! And I was determined to figure out why.
Unravelling the Cobra Effect
SIHUMI is a community where bacteria interact with one another, and understanding these interactions was key to explaining our very own cobra effect.
To investigate this, we used a cross-streak assay—a simple method where we streak different SIHUMI members perpendicularly on agar to observe how they influence each other’s growth. This revealed that some community members naturally inhibit others. By mapping these interactions, we created the inter-species interaction network for SIHUMI. Through this, we discovered that many members of the community naturally inhibit C. difficile growth. In other words, the community already does a pretty good job of keeping C. difficile levels in check.
When we analyzed how our bacteriocin-producing strain affected other community members, we found that it targeted some of C. difficile’s natural inhibitors. This relieved the antagonistic pressure on C. difficile, ultimately allowing it to thrive. So, even though our bacteriocins targeted C. difficile directly, they also disrupted its natural inhibitors, tipping the balance in C. difficile’s favor.
Lessons Learned
This experience taught us that when tackling C. difficile within the gut microbiome, specificity is just as important as potency. Preserving the integrity of the microbial community is essential to prevent C. difficile overgrowth and avoid a "cobra effect."
We also learned that microbiome models are critical when assessing the efficacy of antimicrobials. Tests against single-target pathogens can overestimate an antibiotic’s inhibitory potential because they fail to capture the complex dynamics of a microbial community.
And yes, we know that SIHUMI is a simplified model and has limitations in replicating the complexity of the human gut. However, its simplicity allows us to track individual members and analyze their interactions, providing valuable insights into why broad-spectrum antimicrobials often fail to control C. difficile infections.
Closing Thoughts
This roller coaster of emotions has been a humbling experience. It served as a reminder that even the best-intentioned solutions can backfire in unexpected ways. We need to keep searching for tailored, specific antimicrobial solutions while ensuring we use the right tools to study the microbiome. Who would have thought an anecdote about cobras could shed some light on the fate of C. difficile in the gut?
References
Ríos Colombo, N. S., Ross, R. P., & Hill, C. (2025). “Synergistic and Off-Target Effects of Bacteriocins in a Simplified Human Intestinal Microbiome: Implications for Clostridioides difficile Infection Control” Gut Microbes. http://dx.doi.org/10.1080/19490976.2025.2451081.
Ríos Colombo, N. S., Perez-Ibarreche, M., Draper, L. A., O’Connor, P. M., Field, D., Ross, R. P., & Hill, C. (2023). Impact of bacteriocin-producing strains on bacterial community composition in a simplified human intestinal microbiota. Frontiers in Microbiology, 14. https://doi.org/10.3389/fmicb.2023.1290697
Sugrue, I., Ross, R. P., & Hill, C. (2024). Bacteriocin diversity, function, discovery and application as antimicrobials. Nature Reviews Microbiology, 1–16. https://doi.org/10.1038/s41579-024-01045-x
Walsh, L., Lavelle, A., O’Connor, P., Hill, C., & Ross, R. P. (2024). Comparison of fidaxomicin, thuricin CD, vancomycin and nisin highlights the narrow spectrum nature of thuricin CD. Gut Microbes, 16(1), 2342583. https://doi.org/10.1080/19490976.2024.2342583