Gastrointestinal Motility: The Dynamic Conductor of the Gut Ecosystem
By Benjamin Levine
After a busy day of conducting experiments, caring for patients, or ensuring your kids arrive at school on time, you finally sit down to enjoy a nutritious meal. After a few bites of food, you begin to chew a bit slower, squint your eyes, and wonder: how long will this food be inside me? How does my intestine know how to respond to this? You glance at your partner, wondering if their gut responds to the meal similarly to yours. As a PhD student investigating the nuances of this phenomenon, this scenario occurs quite frequently!
The coordinated movement of food and other gut contents throughout the intestinal tract, a process called intestinal motility, is shaped by our environment, which, in turn, shapes our physiology. What did you eat today, and what is your dietary pattern? Are you physically active, hydrated, well-rested, ill, or stressed? All of these environmental factors shape intestinal motility differently in different people.
Scientists study intestinal motility in specific contexts, like after a meal, in vivo using techniques like magnetic resonance imaging (MRI), manometry, ingestible wireless electronic capsules, surrogate markers like colored dyes, ex vivo using organ baths, or in vitro using enteric neurons for patch clamp assays (1-4). Thus far in my PhD program in nutritional sciences, I’ve become familiar with some of these techniques! Despite its importance, intestinal motility remains an underappreciated regulatory factor in nutritional and microbiome sciences.
Why is intestinal motility so important, and how is it regulated?
At its core, nutrition encompasses how organisms acquire nutrients to facilitate growth, maintenance, reproduction, and survival—like a butterfly sipping nectar or enjoying your favorite meal. Once acquired, your forkfuls of food are digested, absorbed into the body by the gut, and mobilized throughout the body. Intestinal motility is widely recognized as the physiological process that connects nutrient digestion and absorption to the postprandial appearance of nutrients in the bloodstream. Yet, the importance of intestinal motility in regulating whole-body physiology extends far beyond nutrient digestion and absorption.
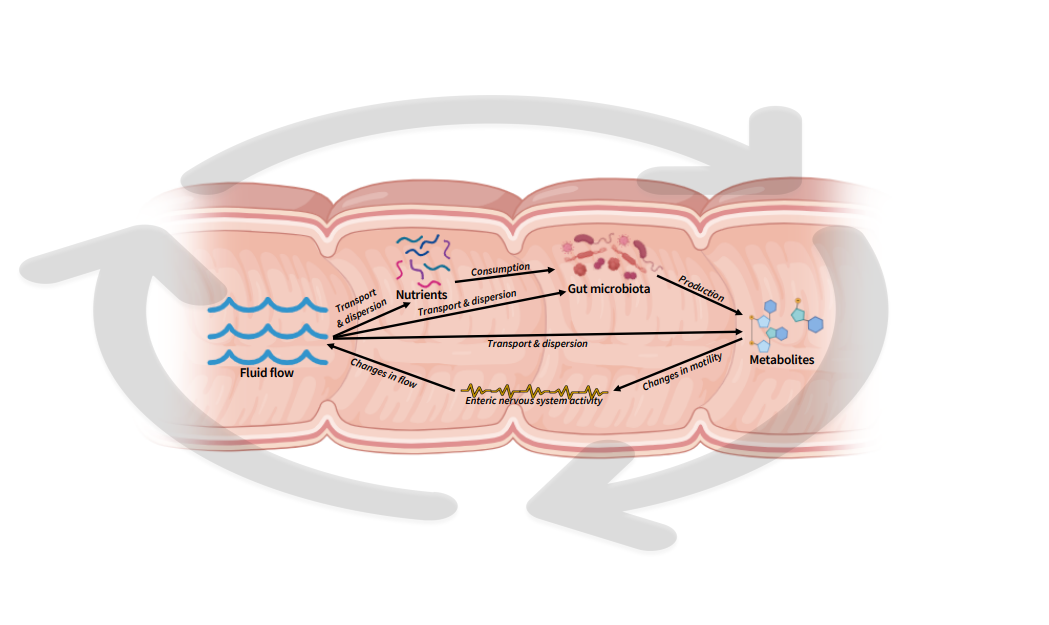
Intestinal motility orchestrates the entire gut ecosystem! This is facilitated by an elaborate network of enteric neurons and glia, smooth muscle cells, interstitial cells, enteroendocrine cells, the gut microbiota, and their metabolites. The intestine’s independent nervous system, the enteric nervous system, operates autonomously to coordinate intestinal motility through contraction and relaxation responses; and is maintained by the local microbiota (1,5). Intestinal motility dictates the exposure of luminal components (fluid, nutrients, gut microbes, microbial metabolites, and host metabolites), to one another and the host epithelium (Figure 1). Hence, intestinal motility is a critical component regulating diet-host-microbe interactions.
Figure 1: Visualization illustration of fluid-nutrient-microbe-metabolite-motility dynamics within the intestinal tract. Created with Biorender.
How do we even begin to predict and leverage these interactions?
Gaining mechanistic insights into how intestinal motility shapes digestion, absorption, and gut microbiota is highly challenging. This is due to the limitations of current approaches in capturing transient changes in the gut environment in vivo over time. The complexity of these interactions is visually simplified in Figure 1, where gut microbes, microbial metabolites, and nutrients are transported, mixed, and dispersed by the gut's fluid flow. Thus, the stability of the microbial population is greatly influenced by the flow. Simultaneously, flow is driven by intestinal motility, which is affected by microbial metabolites and host-related factors such as circadian rhythms, meal composition, and luminal content viscosity (Figure 2) (1). It makes your head spin and your gut wretch, doesn’t it?
How do we measure intestinal motility in the lab?
For my doctoral thesis, I am *attempting* to disentangle how prebiotic fiber consumption and psychological stress exposure shape intestinal motility via host-microbe interactions using pre-clinical and clinical models. As the first PhD student to join my PI’s lab, I was the first to coordinate and conduct both a mouse and human trial. While this task presented unique challenges and stressors, I embraced and appreciated the opportunity. I am grateful to my PI for entrusting me with this leadership role and allowing me to taste-test the experimental meals for the clinical trial! I am *yet* to try the rodent diets...
Figure 2: One of my goals for 2025 is to consume this 25lb bag of 5-grain (whole wheat, rye, barley, oats, triticale) hot cereal that’s packed with various prebiotic, viscous, whole-grain fibers. I enjoy adding various fruits, nuts, and seeds to further enhance the fiber diversity!
Using mice as a pre-clinical model, we conducted a controlled feeding study employing prebiotic fibers with varying structures. We hypothesized this would differentially shape the intestinal environment across intestinal segments, enabling specific microbes to produce a metabolite of interest. I am being purposefully vague because this work is *soon to be* published (fingers crossed). We quantified the concentration of our metabolite of interest in luminal contents to find a physiologically relevant concentration. Then, we incubated intestinal tissue ex-vivo to the physiologic concentrations in an organ bath transducer system (3). Mouse intestinal tissue is mounted in a temperature-controlled physiological solution chamber and attached to a force transducer, which detects changes in tension as the tissue contracts or relaxes in response to metabolite exposure. Although organ baths lack the complexity of an intact organism, they offer valuable insights into how microbial metabolites influence intestinal motility in a tightly controlled environment.
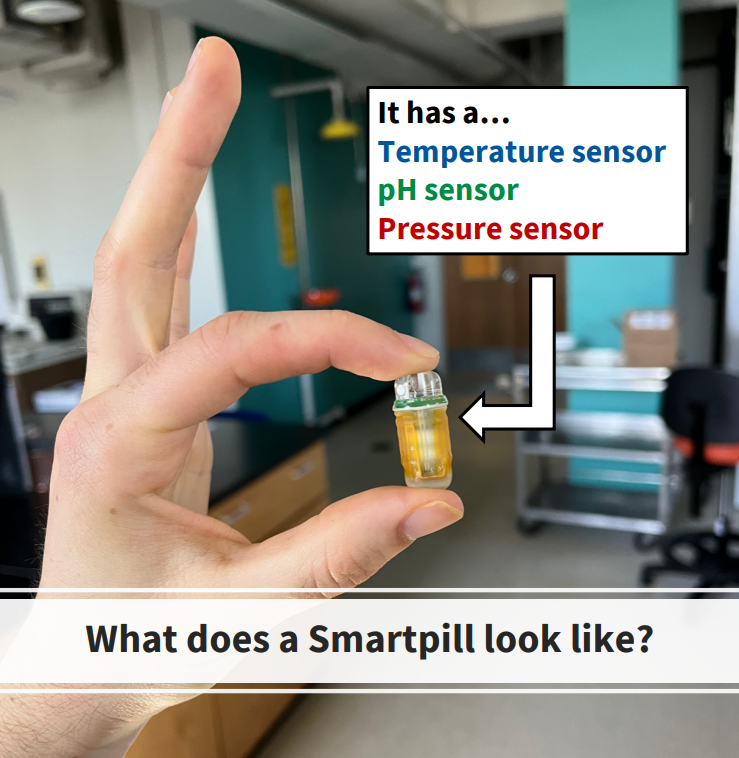
Most recently, we conducted a clinical trial using Smartpills (Figure 3): ingestible, wireless motility capsules that traverse the intestinal tract, recording pH, pressure, and temperature with high granularity. These data are transmitted to an external receiver for downstream analysis after the participant defecates the Smartpill (4).
Figure 3: Ben holding a faulty Smartpill.
We aim to identify individualized patterns in intestinal motility and the gut environment in response to acute feeding of (tasty) prebiotic fibers! This technology was originally developed for diagnostic testing in gastroenterology clinics to assess individuals suffering from severe intestinal dysmotility. However, leveraging this technology for research has been fruitful for us and other labs globally (6-8).
Closing Remarks
Although the knowledge gap in understanding how diet-host-microbe interactions shape intestinal motility is *slowly* narrowing, enormous efforts and novel technologies are still required to untangle the underlying mechanisms. Ultimately, this progress could enable the application of microbiota-targeted nutritional therapies and personalized recommendations in clinical practice and your busy everyday life. Until then, sit, relax, and enjoy your food!
References
Waclawiková, Barbora, Agnese Codutti, Karen Alim, and Sahar El Aidy. “Gut Microbiota-Motility Interregulation: Insights from in Vivo, Ex Vivo and in Silico Studies.” Gut Microbes 14, no. 1 (n.d.): 1997296. https://doi.org/10.1080/19490976.2021.1997296.
Asnicar F, Leeming, ER, Dimidi, E, Mazidi, M, Franks, P, Khatib, HA, Valdes, AN, Davies, R, Bakker, E, Francis, L, et al. Blue poo: impact of gut transit time on the gut microbiome using a novel marker. Gut. 2021:1–10.
Wattchow, David A., Simon J. Brookes, Nick J. Spencer, Paul T. Heitmann, Roberto De Giorgio, Marcello Costa, and Phil. G. Dinning. “From the Organ Bath to the Whole Person: A Review of Human Colonic Motility.” ANZ Journal of Surgery 94, no. 3 (2024): 320–26. https://doi.org/10.1111/ans.18779.
Cummins, G. “Smart Pills for Gastrointestinal Diagnostics and Therapy.” Advanced Drug Delivery Reviews 177 (October 1, 2021): 113931. https://doi.org/10.1016/j.addr.2021.113931.
Vicentini, F.A., Keenan, C.M., Wallace, L.E. et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 9, 210 (2021). https://doi.org/10.1186/s40168-021-01165-z
Pirkola, Laura, Reijo Laatikainen, Jussi Loponen, Sanna-Maria Hongisto, Markku Hillilä, Anu Nuora, Baoru Yang, Kaisa M Linderborg, and Riitta Freese. “Low-FODMAP vs Regular Rye Bread in Irritable Bowel Syndrome: Randomized SmartPill® Study.” World Journal of Gastroenterology 24, no. 11 (March 21, 2018): 1259–68. https://doi.org/10.3748/wjg.v24.i11.1259.
Procházková, Nicola, Martin F. Laursen, Giorgia La Barbera, Eirini Tsekitsidi, Malte S. Jørgensen, Morten A. Rasmussen, Jeroen Raes, Tine R. Licht, Lars O. Dragsted, and Henrik M. Roager. “Gut Physiology and Environment Explain Variations in Human Gut Microbiome Composition and Metabolism.” Nature Microbiology 9, no. 12 (December 2024): 3210–25. https://doi.org/10.1038/s41564-024-01856-x.
Corbin, Karen D., Elvis A. Carnero, Blake Dirks, Daria Igudesman, Fanchao Yi, Andrew Marcus, Taylor L. Davis, et al. “Host-Diet-Gut Microbiome Interactions Influence Human Energy Balance: A Randomized Clinical Trial.” Nature Communications 14, no. 1 (May 31, 2023): 3161. https://doi.org/10.1038/s41467-023-38778-x.