Can Postbiotics Combat Colitis? Insights from Mouse Models
By Chrysanthos Kleanthous
The emergence of postbiotics as a new approach for improving health is raising much interest, although the health benefits conferred by non-viable microbes are not a new concept. In particular, their potential to manage inflammatory bowel disease (IBD) has been the focus of many animal and human studies. As part of my BSc research project in the School of Biological Sciences (University of Reading, UK), I had the opportunity to conduct a systematic literature review and meta-analysis on this subject. My specific objective was to investigate the effects of postbiotics on chemically-induced colitis (CIC) in mice.
IBD is a group of chronic relapsing disorders, including Crohn’s disease and ulcerative colitis, that cause inflammation of the gastrointestinal tract (Cai et al. 2021). The ‘’European Federation of Crohn’s and Ulcerative Colitis Associations’’ (EFCCA) suggests that IBD may affect as many as 10 million people globally (EFCCA, 2023). Affected individuals experience a range of symptoms, including abdominal pain, diarrhoea, and weight loss, significantly reducing their quality of life. The current treatments for IBD include surgery and pharmacotherapy, but these are associated with undesirable effects and complications. Therefore, alternative treatment options for managing human IBD are still required. One such option which has the potential to effectively treat human IBD involves the improvement of intestinal microecology through the administration of agents belonging to the ‘-biotics’ family of substances (e.g., probiotics, prebiotics and postbiotics).
Postbiotics are defined by the ‘International Scientific Association of Probiotics and Prebiotics’ (ISAPP) as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” (Salminen et al., 2021). In simpler terms, postbiotics are non-viable microorganisms and/or their cell components, with or without their metabolites, that confer a health benefit. However, when it comes to precisely defining postbiotics, things could get challenging, especially if definitions published earlier are considered. For instance, many articles refer to purified metabolites as ‘postbiotics’, which conflicts with ISAPP’s definition. Therefore, to maintain consistency and accuracy in our research, only studies that met the criteria of ISAPP’s postbiotic definition were considered for inclusion.
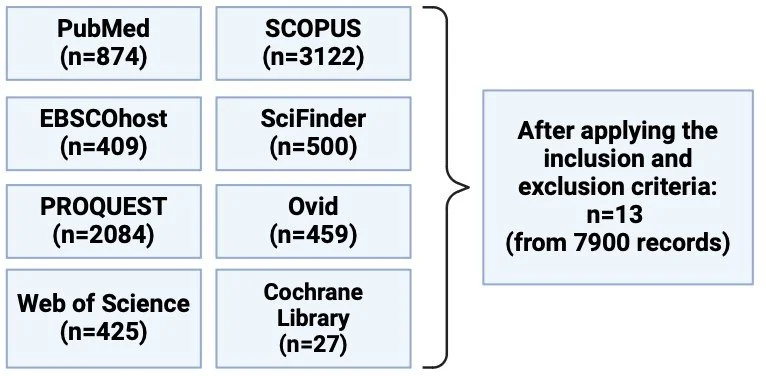
Initially, a systematic search of eight databases was performed to identify all the relevant papers, followed by the application of inclusion and exclusion criteria which resulted in the selection of 13 papers (Figure 1). Note that colitis was induced by oral (or rectal) treatment of otherwise healthy mice with chemicals such as dextran sodium sulphate, which induce human-like colitis.
Figure 1. To identify the eligible articles for the meta-analysis, eight databases were searched which identified a total of 7900 records. After applying the inclusion and exclusion criteria, 13 articles were eligible for inclusion. The inclusion criteria were: 1) Primary research articles only, 2) Mice studies only (no age, sex, weight, strain restrictions), 3) Inactivated microbiological biomass was required to be present during the progression of the experiment (based on the ISAPP’s postbiotic definition). Studies that used purified metabolites were excluded.
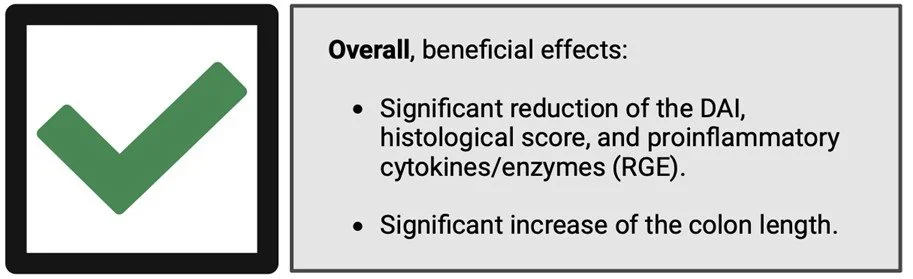
The extracted data allowed us to statistically assess the effects of postbiotics on CIC in mice using 7 distinct parameters: CIC disease severity (disease activity index [DAI], histological score, colon length); proinflammatory cytokine/enzyme levels (IL-1β, IL-6, TNF-α, COX-2) (Figure 2). Overall, the research highlighted the efficiency of postbiotics in alleviating CIC in mice as determined by examination of the immunological, biochemical, and histological changes, confirming the initial hypothesis. Most of the articles (11/13) used a heat-inactivation step for postbiotic preparation and all used bacteria that belong to one of the following genera: Lactobacillus, Enterococcus, Faecalibacterium, Bifidobacterium and Lactiplantibacillus.
Figure 2. The beneficial effects of postbiotics on CIC in mice. RGE: relative gene expression (of the proinflammatory cytokines/enzymes); IL-1β, IL-6, TNF-α, COX-2).
The disease activity index (DAI) evaluates the clinical progression of colitis using a composite score system. Generally, it is calculated as the sum of body weight loss, faecal properties, and haematochezia scores. Our meta-analysis suggests a significant reduction in the DAI in mice with CIC upon postbiotic administration, compared to the untreated group. Additionally, the postbiotic-administered group showed a significant reduction in the histological score. Cumulatively, these findings highlight the effects of postbiotics on reducing the severity of CIC in mice. Moreover, our research underlined the impact of postbiotics in lowering the relative gene expression (RGE) of various proinflammatory cytokines, including IL-1β, IL-6 and TNF-α. Indeed, postbiotics were found to have important effects in suppressing inflammation within the colonic mucosa. For instance, Choi et al. (2019) suggested that crypt destruction is attenuated in the postbiotic-administered group compared to their colitis-only group.
Overall, our systematic review indicates that postbiotics are promising agents for potential use in treating IBD. However, it is important to note that our research was solely based on mouse studies and therefore the reported associations still require verification in humans. Future research and further clinical investigations are required before postbiotics can be considered as feasible therapeutic agents for people who suffer from IBD.
The above study was performed during the final year of my BSc Microbiology degree (2022/23). With the help of my supervisors (Professor Simon Andrews, Dr. Natasha Barrett) and Dr. Gabriel Vinderola (ISAPP board member), my dissertation was converted into a manuscript which was submitted for consideration for publication in October 2023, and is currently under peer-review assessment. Stay tuned!
References:
Cai, Z., Wang, S., & Li, J. (2021). Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Frontiers in Medicine, 8.
Choi, E. J., Lee, H. J., Kim, W. J., Han, K. Il, Iwasa, M., Kobayashi, K., Debnath, T., Tang, Y., Kwak, Y. S., Yoon, J. H., & Kim, E. K. (2019). Enterococcus faecalis EF-2001 protects DNBS-induced inflammatory bowel disease in mice model. PLoS ONE, 14(2).
EFCCA. (2023). What is IBD? European Federation of Crohn’s and Ulcerative Colitis Associations. Available at: https://efcca.org/content/what-ibd (Accessed: 22 Feb 2024).
Salminen, S., Carmen Collado, M., Endo, A., Hill, C., Lebeer, S., M Quigley, E. M., Ellen Sanders, M., Shamir, R., Swann, J. R., Szajewska, H., & Vinderola, G. (2021). The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nature Reviews Gastroenterology and Hepatology, 18, 649–667.